


Next: About this document ...
Up: Labs and Projects for
Previous: Labs and Projects for
Subsections
The purpose of this lab is to use Maple to study applications of
exponential and logarithmic functions. These are used to model many
types of growth and decay, for example bacterial growth and
radiaoctive decay. This lab also describes applications of exponential
and logarithmic functions for heating and cooling and to medicine dosage
The simple model for growth is
exponential growth, where
it is assumed that
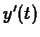 is proportional to
is proportional to 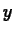 . That is,
. That is,
Separating the variables and integrating (see section 4.4 of the text),
we have
so that
In the case of exponential growth, we can drop the absolute value
signs around 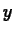 , because
, because 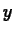 will always be a positive quantity.
Solving for
will always be a positive quantity.
Solving for 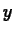 , we obtain
, we obtain
which we may write in the form 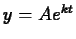 , where
, where 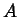 is an
arbitrary positive constant.
is an
arbitrary positive constant.
In a sample of a radioactive material, the
rate at which atoms decay is proportional to the amount of material present.
That is,
where 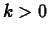 is a constant. This is the same equation as in exponential growth,
except that
is a constant. This is the same equation as in exponential growth,
except that 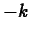 replaces
replaces 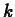 . The solution is
. The solution is
where 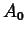 is a positive constant. Physically,
is a positive constant. Physically, 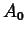 is the amount of
material present at
is the amount of
material present at 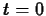 .
.
Radioactivity is often expressed in terms of an element's half-life.
For example, the half-life of carbon-14 is 5730 years. This statement means
that for any given sample of
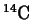 , after 5730 years, half of it
will have undergone decay.
So, if the half-life is of an element Z is
, after 5730 years, half of it
will have undergone decay.
So, if the half-life is of an element Z is 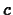 years, it must be
that
years, it must be
that
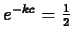 , so that
, so that 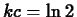 and
and
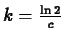 .
.
What is usually called Newton's law of cooling is a simple model for
the change in temperature of an object that is in contact with an
environment at a different temperature. It says that the rate of
change of the temperature of an object is proportional to the
difference between the object's temperature and the temperature of the
environment. Mathematically, this can be expressed as the differential
equation
where 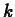 is the constant of proportionality and
is the constant of proportionality and
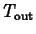 is
the temperature of the environment. Using a technique called
separation of variables it isn't hard to derive the solution
is
the temperature of the environment. Using a technique called
separation of variables it isn't hard to derive the solution
where 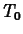 is the temperature of the object at
is the temperature of the object at 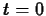 .
.
The main functions you need are the natural exponential and
natural logarithm. The Maple commands for these functions are
exp and ln. Here are a few examples.
>
f := x -> exp(-2*x);
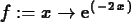
>
simplify(ln(3)+ln(9));
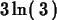
>
ln(exp(x));
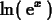
>
simplify(ln(exp(x)),assume=real);
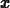
The assume=real is needed in the command above, because Maple
usually works with complex variables.
>
solve(exp(-3*x)=0.5,x);
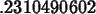
>
plot(log[10](x),x=0..100);
Sometimes you need to use experimental data to determine the value of
constants in models. For example, suppose that for a particular drug,
the following data
were obtained. Just after the drug is injected, the concentration is
1.5 mg/ml (milligrams per milliliter). After four hours the
concentration has dropped to 0.25 mg/ml. From this data we can
determine values of 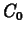 and
and 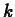 as follows. The value of
as follows. The value of 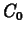 is the
initial concentration, so we have
is the
initial concentration, so we have
To find the value of 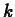 we need to solve the equation
we need to solve the equation
which we get by plugging in 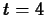 and using the data
and using the data
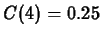 . Maple commands for solving for
. Maple commands for solving for 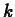 and defining and
plotting the function
and defining and
plotting the function 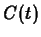 are shown below.
are shown below.
>
k1 := solve(0.25=1.5*exp(-4*k),k);
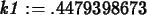
>
C1 := t -> 1.5*exp(-k1*t);
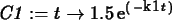
>
plot(C1(t),t=0..6);
- In 1935 Charles F. Richter of Cal Tech developed a scale for
measuring the magnitude of earthquakes. The Richter Scale formula is
given by
where 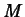 is the magnitude of the earthquake,
is the magnitude of the earthquake, 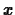 is the amplitude of
the largest seismic wave as measured on a standard seismograph 100
kilometers from the epicenter
and
is the amplitude of
the largest seismic wave as measured on a standard seismograph 100
kilometers from the epicenter
and 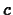 is the amplitude of a reference earthquake of amplitude 1
micron ( 1 micron is 0.001 mm) on a standard seismograph at the same
distance from the epicenter.
is the amplitude of a reference earthquake of amplitude 1
micron ( 1 micron is 0.001 mm) on a standard seismograph at the same
distance from the epicenter.
- In 1989, the San Francisco Bay area suffered severe damage from
an earthquake of magnitude 7.1. However, the damage was not nearly as
extensive as that caused by the great quake of 1906, which has been
estimated to have had the magnitude 8.3. What is the ratio of the
amplitude of the 1906 quake to the 1989 quake?
The largest earthquake magnitude ever measured was
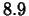 for an
earthquake in Japan in 1933. Determine the ratio of the amplitude of
this earthquake to that of the 1906 San Francisco earthquake.
for an
earthquake in Japan in 1933. Determine the ratio of the amplitude of
this earthquake to that of the 1906 San Francisco earthquake.
- Worldwide use of the Internet is increasing at an exponential
rate. If traffic on the Internet is doubling every 150 days, find the
exponential growth rate constant.
- Suppose that the last bit of ice in a picnic cooler has
melted. How long will it take for the temperature inside to reach
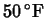 ? Use Newton's law of cooling to model this, using
? Use Newton's law of cooling to model this, using
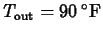 ,
,
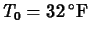 and
and
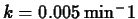 .
.
- The worst nuclear accident in history happened in 1986 at the
Chernobyl nuclear
plant near Kiev in the Ukraine. An explosion destroyed one of the plant's four
reactors, releasing large amount of radioactive isotopes into the atmosphere.
Consider 10 grams of the plutonium isotope Pu-239 released in the Chernobyl
nuclear accident. This isotope has a half-life of 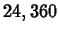 years. How
long will it take for the 10 grams to decay to 1 gram?
years. How
long will it take for the 10 grams to decay to 1 gram?



Next: About this document ...
Up: Labs and Projects for
Previous: Labs and Projects for
Jane E Bouchard
2001-04-16
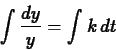
![]() , after 5730 years, half of it
will have undergone decay.
So, if the half-life is of an element Z is
, after 5730 years, half of it
will have undergone decay.
So, if the half-life is of an element Z is ![]() years, it must be
that
years, it must be
that
![]() , so that
, so that ![]() and
and
![]() .
.
![]() and
and ![]() as follows. The value of
as follows. The value of ![]() is the
initial concentration, so we have
is the
initial concentration, so we have
![]() years. How
long will it take for the 10 grams to decay to 1 gram?
years. How
long will it take for the 10 grams to decay to 1 gram?